Abstract
Introduction - Elevated expression of XPO1 (Exportin 1) increases the nuclear export / inactivation of tumor suppressor proteins (e.g. p53, IkB, p21, FOXO) and promotes the export / translation of eIF4E-bound oncoprotein mRNAs (e.g. c-MYC, BCL-2, Cyclin D). Selinexor, the first oral Selective Inhibitor of Nuclear Export (SINE) compound, inhibits XPO1 and has shown promising anti-cancer activity across hematological and solid tumors in Phase 1 and 2 clinical trials, including in patients (pts) with relapsed/refractory multiple myeloma (RRMM). Considering the breadth of knowledge on selinexor ± dexamethasone (dex) in RRMM, eltanexor (KPT-8602), a second-generation oral SINE compound, was evaluated for safety and tolerability in this pt population. The primary objectives were to determine the safety, preliminary anti-MM activity, and the recommended Phase 2 dose (RP2D) of eltanexor ± dex.
Methods - In this Phase 1/2 study, using a 3+3 dose escalation design, eltanexor is dosed orally (QDx5 or QoDx3 weekly) for a 28-day cycle with a starting dose of 5 mg. Pts with less than a minor response (MR) after 1 cycle or partial response (PR) after 2 cycles were permitted to add low dose dex. In some pts, dex was added from day 1. The pharmacokinetic (PK) and pharmacodynamic (XPO1 mRNA) profiles of eltanexor were evaluated.
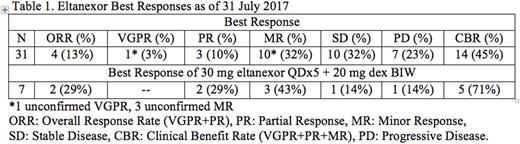
Results - As of 31-July-2017, 36 pts (23 M/13 F; median prior therapies: 7; median age: 66) with RRMM were enrolled; most with quad- or penta-refractory MM. Median duration of treatment is 76 days (range: 10 - 441). Most common drug related adverse events (AEs; >30%, any grade) are thrombocytopenia, nausea, neutropenia, anemia, leukopenia, fatigue, diarrhea, and dysgeusia. Grade ≥3 related AEs include thrombocytopenia (43%), neutropenia (26%), leukopenia (14%), and anemia (11%). Although 2 dose limiting toxicities (DLTs; >4 missed doses and Grade 4 thrombocytopenia) were observed in different cohorts, the protocol-defined maximum tolerated dose (MTD) was not reached. Further evaluation of 20 or 30 mg eltanexor QDx5 + dex is ongoing in expansion cohorts. The preliminary efficacy of eltanexor (Table 1) shows that eltanexor ± dex can induce responses and disease stabilization. A dose proportional PK profile without accumulation has been observed. Mean Cmax and AUC0-inf values for eltanexor doses of 5 - 60 mg ranged from 36.6 - 284 ng/mL and 164 - 2,581 ng*h/mL, respectively. Tmax and t½ ranged from 0.5 - 8.0 h and 4.0 - 7.1 h, respectively. For all cohorts evaluated, the fold-change maximum (Fmax) of XPO1 mRNA expression was achieved 4 - 8 h post-dose (tmax). The Fmax for XPO1 mRNA was roughly dose proportional to the Cmax of eltanexor. As expected, based on observations with other SINE compounds, the plateau for Fmax of XPO1 mRNA in treated pts was ~6 - 10-fold change and was achievable at 40 - 60 mg of eltanexor.
Conclusions - Based on preliminary data, eltanexor is well tolerated and demonstrates promising activity in this heavily pre-treated RRMM population. The gastrointestinal and constitutional toxicities observed with <40 mg eltanexor QDx5 are low grade, transient, and do not limit dosing. Thrombocytopenia (with minimal clinical impact) was the most common hematologic toxicity and was expected in this population. As with selinexor, addition of low-dose dex (20 mg) twice weekly (BIW) to the eltanexor treatment regimen appeared to improve efficacy while maintaining tolerability. Of the 31 evaluable pts, 25 received dex with their eltanexor regimen. Therefore, both 20 and 30 mg eltanexor QDx5 + 20 mg dex BIW are being evaluated to clearly define the RP2D. Based on these results and anti-tumor activity previously observed with selinexor, pts with advanced colorectal cancer, castration resistant prostate cancer, and myelodysplastic syndrome are being enrolled.
Rossi: Thrassos: Consultancy; Celgene: Consultancy. Baz: Sanofi: Research Funding; BMS: Research Funding; merck: Research Funding; takeda: Research Funding; celgene: Honoraria, Research Funding; karyopharm: Research Funding. Hofmeister: Roche: Research Funding; Celgene: Research Funding; Karyopharm: Research Funding; Takeda: Research Funding; Janssen: Research Funding; Thrassos: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Research Funding. Shustik: Amgen: Honoraria; Takeda: Honoraria; Celgene: Honoraria. Richter: Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Speakers Bureau. Chen: Abbvie: Honoraria; Amgen: Honoraria; Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Vogl: Teva: Consultancy; Karyopharm: Consultancy; Constellation: Research Funding; GSK: Research Funding; Takeda: Consultancy, Research Funding; Celgene: Consultancy; Amgen: Consultancy; Calithera: Research Funding. Baloglu: Karyopharm Therapeutics Inc: Employment, Equity Ownership. Senapedis: Karyopharm Therapeutics Inc: Employment, Equity Ownership. Ellis: Karyopharm Therapeutics Inc: Employment, Equity Ownership. Shacham: Karyopharm Therapeutics Inc: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Kauffman: Karyopharm Therapeutics Inc: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal